
Abstract: au88ge12 alloy solder has the advantages of low vapor pressure, low coefficient of thermal expansion, good corrosion resistance, good high temperature stability, good wettability and good thermal conductivity. It is widely used in electronic products and vacuum devices because of its excellent performance. However, the coarse primary phase Au and eutectic structure formed in the as cast structure of au88ge12 lead to the brittleness of au88ge12 eutectic solder at room temperature, which is difficult to process and shape. 0.2wt.% The effect of sb on the modification of Au Ge eutectic alloy. It is proved by metallographic analysis, DSC and other characterization means that the modification is conducive to the refinement of au88ge12 solder structure and improve its machinability, so as to expand the application of au88ge12 solder in the field of electronic packaging.
Keywords: Au Ge eutectic alloy; Metamorphic treatment; Primary phase; Microstructure refinement
Abstract:Au88Ge12 alloy solder has many advantages, such as low vapor pressure, low coefficient of thermal expansion, good corrosion resistance, high temperature stability, good wettability and good thermal conductivity. It is widely used in electronic products and vacuum devices because of its excellent properties. However, the coarse primary Au-phase and eutectic structure formed in the as cast structure of Au88Ge12 lead to the extremely brittle eutectic solder of Au88Ge12 at room temperature, which is difficult to be processed and formed. In this paper, the effect of 0.2wt.% Sb on the modification of Au-Ge eutectic alloy is studied. It is proved by metallographic analysis and DSC that the modification is beneficial to the refinement of the structure of Au88Ge12 solder and the improvement of its machinability, so as to expand the application of Au88Ge12 solder in the field of electronic packaging.
Keywords:Au-Ge eutectic alloy, primary phase, modification, structure refinement
1. Introduction
In some precision instruments in aerospace, electronic communication and other fields, Au based precious metals are often used as solder, and common solder alloys include Au80Sn20, au88ge12 (hereinafter referred to as "Au Ge eutectic alloy") and au97si3. Among them, Au Ge eutectic alloy has the advantages of low vapor pressure, low coefficient of thermal expansion, good corrosion resistance, good high temperature stability, good wettability and good thermal conductivity [1,2]. Because of its excellent performance, it is widely used in electronic products and vacuum devices. Its main advantages are as follows:
(1) High strength and low sealing temperature: the tensile strength of Au Ge eutectic alloy at room temperature is as high as 220MPa, which reaches the level of some high-temperature solder; But at the same time, its eutectic temperature is only 356 °, which provides relatively high sealing strength at low sealing temperature;
(2) Low coefficient of thermal expansion. Electronic devices often undergo repeated thermal cycles during service. The periodic shear stress on the welding surface during thermal cycle is an important reason for package failure. The CTE of Au Ge eutectic alloy (10.3ppm / ℃) is better than that of Au series solder AuSn (16ppm / ℃) and Ausi (13ppm / ℃);
(3) Good wettability and corrosion resistance. Au Ge eutectic alloy has good fluidity and wettability, and there is no etching phenomenon for gold plating layer. Its alloy content is 88WT% Au, which is close to the composition of gold plating layer, and the immersion degree of thin coating through diffusion is very low;
2. Modification of Au Ge eutectic alloy
When the eutectic temperature of Au Ge alloy is 356 ℃, the solid solubility of Ge in Au is ≤ 0.1wt% Ge. Although the atomic radius of Ge is close to that of Au, it is basically insoluble in Au and exists in the form of single crystal. When the content of Au is 72at% and Ge is 28AT%, it is the eutectic point. The binary phase diagram [3] of Au Ge alloy is shown in Fig. 1. Generally, due to the deviation of Au Ge eutectic alloy from equilibrium solidification, complete eutectic structure can not be obtained. Coarse primary phase Au and eutectic structure are generated in the as cast structure. Therefore, Au Ge eutectic alloy is very brittle at room temperature. Even under the condition of hot rolling, it is still Explosive Edge cracking and difficult to process into thin strip.


4.2 eutectic morphology and composition analysis of Au Ge eutectic alloy
The microstructure of the eutectic structure of the two groups of Au Ge eutectic alloys was observed at 4000 times with a wave spectrometer (WDS) equipped with electron probe, as shown in Fig. 3. For the Au Ge eutectic alloy modified with 0.2wt% Sb, two different eutectic structures appear in the metallographic photos, as shown in (b) and (c) in the figure.

Fig. (a) microscopic morphology of eutectic structure of unmodified Au Ge eutectic alloy. In the figure, the white matrix is composed of Au rich solid solution or pure Au, and the black particle is pure Ge, which is mainly spherical or short rod, with uniform distribution and size of 0.3 μ m~1.0 μ M, the average size is about 0.8 μ m。 Figures (b) and (c) show the microstructure of eutectic in different regions modified with 0.2wt% sb. It can be seen from figure (b) that compared with (a), its black particles become extremely fine, (b) in the figure, they are evenly distributed in spherical and thin strips, with an average size of 0.3 μ m. And light gray flake ausb2 phase appears in the phase diagram. Figure (c) is similar to (a) and (b), but the black particles become more uniform and fine, and the average size is between (a) and (b).
In order to further analyze the effect of modification on Au Ge eutectic alloy, the composition of areas (a) and (b) in Fig. 4 was detected by X-ray energy dispersion spectrometer (EDX) equipped in electron probe.
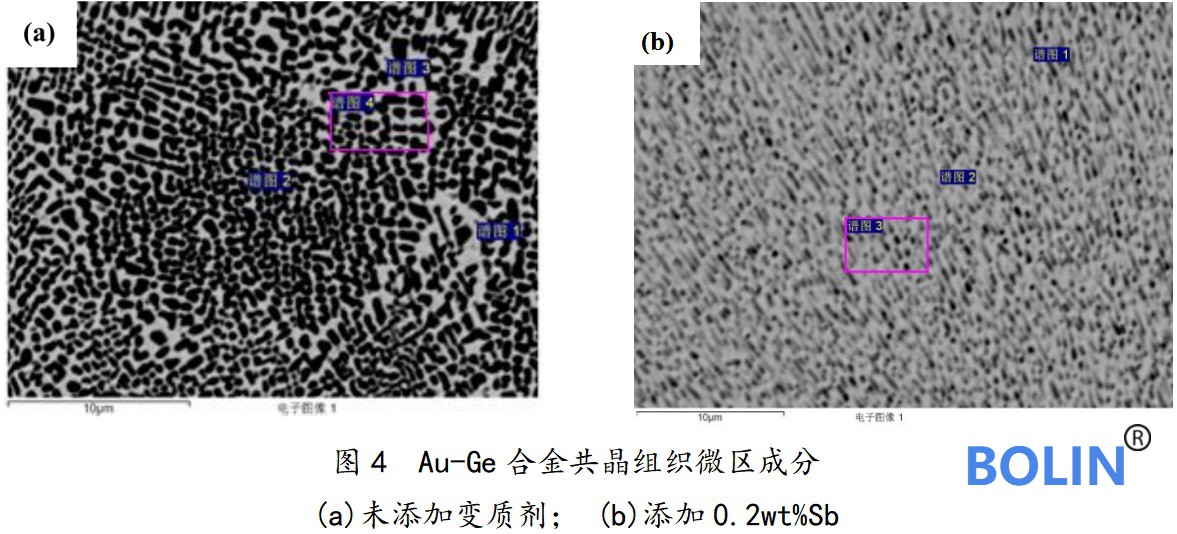
The spectrum test results are shown in Table 1 below:
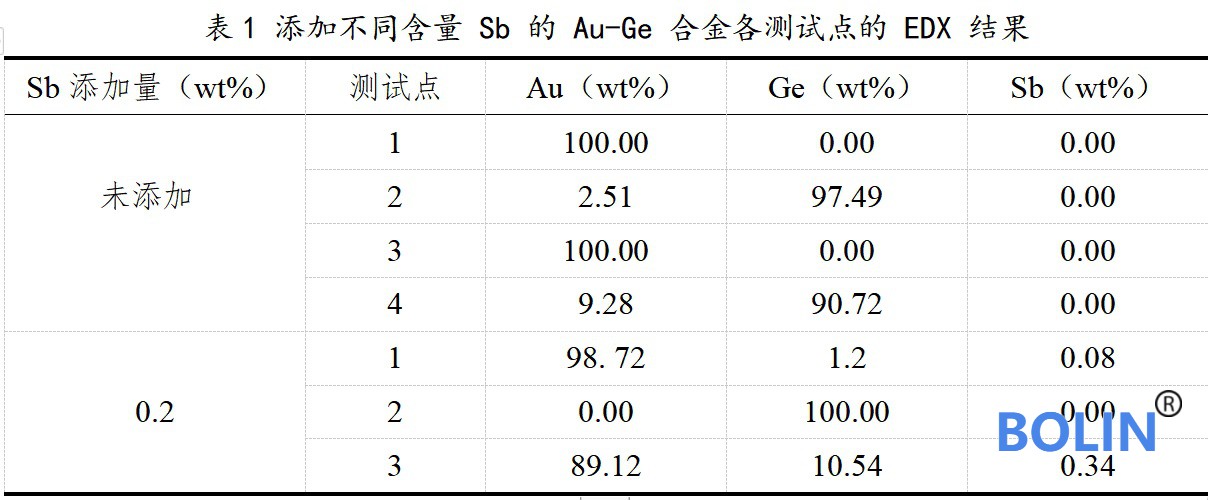
According to the spectrum analysis results in the table, the addition of sb has little effect on the composition of eutectic structure and has no obvious change. Compared with spectrum 4 in (a) and spectrum 3 in (b), the composition of eutectic alloy modified with 0.2wt% Sb is closer to the ideal au88ge12 eutectic composition.
4.3 DSC test of Au Ge eutectic alloy
Add 0.2wt.% to the sample without modifier and The Au Ge eutectic alloy of sb was tested by DSC, and the effect of modifier on the melting properties of Au Ge eutectic alloy was analyzed. Fig. 5 shows the DSC curves of melting characteristics of two groups of Au Ge eutectic alloy samples.

It can be seen from the DSC curve in the figure above that the solidus temperature of Au Ge eutectic alloy without modifier is 368.3 ℃, the liquidus temperature is 372.3 ℃ and the melting temperature range is 4.0. Compared with the above, the solidus temperature and liquidus temperature of Au Ge eutectic alloy with 0.2wt% Sb are reduced by 1.7 ℃ and 1.5 ℃ respectively, and the melting temperature range is not significantly different from the former.
5. Conclusion
The addition of trace Sb has little effect on the composition of eutectic structure, but can significantly improve the microstructure and properties of Au Ge eutectic alloy. The addition of sb "breaks" the strip like primary phase of Au Ge eutectic alloy, and its eutectic structure is significantly refined. The average size of Ge particles in eutectic structure changes from 0.8 μ M becomes 0.3 μ m。 The overall composition of Au Ge eutectic alloy treated with sb modifier is more uniform, and its solid-liquid phase line is closer to the eutectic point.
reference
[1] Xie Hongchao, Yang Anheng Effect of Ni on Microstructure and properties of auge12 alloy [J] Precious metals, 2008, 32 (1): 35-39
[2] Yang Mengli, Zhang Hongxin, Yu Xinming Preparation of ohmic contact in Au Ge system [J] Semiconductor intelligence, 2001, 38 (4): 32-34
[3] Lin H L, Sidat Senanayake, Keh-Yung Chen. Optimization of AuGe-Ni-Au ohmic contacts for
GaAs MOSFETS[J]. IEEE Transactions on Electron Devices, 2010, 50(4): 80-84.
[4] Fan Xiaoming Metal solidification theory and technology [M] Wuhan University of Technology Press, 2012, 11 (6): 193